Well here we go again! Now that the fourth marking period is starting new blogs will soon be coming.
Sodium, Neon, and Silly Cats

Puns are always available in chemistry!
Group one elements first so: SODIUM! As I literally just said, sodium is a group one element and therefore is often called an alkali metal. Although salt is the most common form of sodium [sodium chlroide], it is also used in items such as soap! I never knew that before. Its symbol is Na and has an atomic number of 11.
Neon! Neon is next as it exists in only a small amount of the atmosphere 9 present at 1 part in 65000) naturally as opposed to sodium which everyone has heard of. Ne has 10 protons and electrons and is located in group 18. Most people have heard of Neon colors, but never thought of it as an individual element surrounding us every day!
https://www.webelements.com/sodium/
“Wow, Lindsey’s…
“Wow, Lindsey’s blog was super helpful! It can be found here http://chemistry2013-14.tumblr.com/post/75624209704/relating-it-back-to-chemistry-cotton-candy
I loved reading and learning about my favorite carnival treat. It seems like cotton candy is a relatively easy process, but also complex in its own way. The reason cotton candy works though is because of one thing: chemistry.
The main (and only) ingredient in cotton candy is sugar. One type of sugar is sucrose and it is a carbohydrate. Sucrose is made up primarily of carbon, oxygen, and hydrogen atoms (C12H22O11). A Lindsey pointed out, we learned how different typed of atoms can make up an element.
This sugar is then heated in a cotton candy machine. Once it is heated, the sugar comes to its melting point and the bonds of the constituent molecules break. Then the oxygen and hydrogen within the sugar rearranges to become water, and then evaporate. This leaves behind only the carbon, which begins to burn. After that, the sugar caramelizes.
The hot cotton candy maker continues spinning the hot liquid, and then once it is time to put on the stick, all the microscopically thin strands of liquid sugar cool in the air and become the cloud of sweet puff we enjoy! It’s that simple. But it would not be possible without chemistry!
I used this article to help with my further research on the topic http://theraptorlab.wordpress.com/2013/10/21/the-science-of-cotton-candy/”
Well Megan talked about Lindsey’s post so I wanted to add onto Megans response! This post follows cotton candy and how it is made. ALthough it does describe how to make cotton candy, i remembered hearing that it was dyed and became curious onto how this was done. The answer was simple enough: food coloring! One website I found showed how to make your own colored sugar which I believe is what is used to create cotton candy.
The link is here:
http://www.instructables.com/id/Creating-Custom-Sugar-Colors/
But it still didnt show me what food coloring is made of. For that I looked on the fda website. It provided information such as that the color can sometimes be used to preserve freshness but is mainly used for our own enjoyment because it improves appearance. I mean bright pink and blue candy is definitly more appealing to little kids than a plain white one.
Here: http://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm094211.htm
Overall it was interesting reading the two posts as I would not have thought about the chemistry behind cotton candy without them.
Copper and Tellurium

Oh dear flirty chemists!
Lets begin with copper or Cu! Copper is the 29th element on the periodic table, meaning it as twenty nine protons and electrons. It is in group eleven and is a very important metal. It is also a good conductor of heat and electricity as most metals are. It even gave policemen their nickname of ‘cops’ or ‘coppers’ since their uniforms used to have copper buttons!
Tellurium, Te, has a white or silver appearance. It has an atomic number of 52 meaning 52 protons and electrons. It belongs in group 16 on the periodic table and is semi-metallic. With light exposure, Tellurium becomes a better conductor. Tellurium gives off a garlic like smell.
https://www.webelements.com/copper/
http://www.webelements.com/tellurium/
Silly Putty!
“I’m going to get to the point quickly here- if you didn’t have silly putty as a child, you had a bad childhood. Silly putty is that toy that everyone had, that was also messy and kind of useless. But at the time it was fun. Yet, most of us never understood the chemistry that went along with our favorite childhood toy. So this article explains it all! http://www.chemistryislife.com/site/thechemistryofblank/t
Silly Putty is made up of Silicon Oil, Boric Acid, 65%: Dimethyl Siloxane, 17%: Silica, quartz crystalline, 9%: Thixotrol ST, 4%: Polydimethylsiloxane, 1%: Decamethyl Cyclopentasiloxane, 1%: Glycerine, and 1%: Titanium. However, the main to ingredients are silicon oil and Boric acid. Silicon is a very common element in nature, but silicon oil is man made. It repels water, doesn’t decompose at high temperature, is not toxic, and it doesn’t conduct heat. Boric acid (H3BO3) is colorless (white) and is soluble in water. So when these two things are combined, they make a stretchy, bouncy, sticky material: Silly putty! Its the perfect combination for entertaining a child!”
The above came from Megan’s chemistry blog! While I was looking on it I saw silly putty, and I mean who wouldn’t stop to investigate a psot about silly putty? It was interesting hearing all the unpronouncable ingredients.
Dyes Turning Yellow River Pink

The Yellow River is going pink? Because of tofu? WHAT? I was planning to write a post about water color which can be used to determine the surrounding environments pollution levels, when I came across this little piece of information. As a vegetarian (and tofu lover) I was very curious to see why tofu would need dye, for one thing, and why it is being released into a river in such a way that it is considered pollution.
A stretch of this river located in China has changed to a magenta color due to colors that spill into it almost every day. In this article, unlike the first one I saw, the pinkish color is due to an actual pink dye being released from a steam heating station where water is dyed pink to distinguish it from drinking water.
Ill update on this later, but it does not seem as if many articles want to discuss dyes being released into the river.
http://content.time.com/time/world/article/0,8599,1550046,00.html
Coal Washing Chemical Spill

ALthough I have never heard of washing coal, apparently water doesn’t cut it. Instead of plain, pure water, 4-methylcyclohexane methanol is used. Called MCHM, this chemical helps remove the burnable fossil-fuel from the unburnable products around it like rock and dirt. Thousands of this litters were spilled this past week into the Elk River in West Virginia. 300,000 people are currently advised in the area not to drink the water, which is a rather scary thought. I mean if we can’t drink it, should we really breath in remains of it as the coal is burned? At the moment the toxicity levels are unknown. Within the same article, it states that although MCHM has not been proven to have a large health effect, only eye and skin irritation, it has also not been highly tested for its safety. I suppose this will have to change now that people will easily have contact with this organic solvent.
Can you drink to much water?
Apparently yes! While water has very good properties and is good for you in proper amounts, to much can cause physical issues. Water intoxication for example could be a result which may end in death. this condition occurs when too much water is taken into the body to fast and dilutes the necessary nutrients preventing them from performing their proper functions. As shown in all aspects of chemistry, the body likes to keep a certain balance of good things to prevent it from causing damage. We’ve all heard the phrase to much of a good thing can be bad. Each individual’s amount of water needed to cause water intoxication varies so the exact amount is impossible to know.
I know a lot of people don’t like drinking water because many find it flavorless but I do! Its weird hearing something necessary for survival could also lead to death!
http://health.howstuffworks.com/diseases-conditions/death-dying/water-intoxication.htm
Baking Soda vs Baking Powder
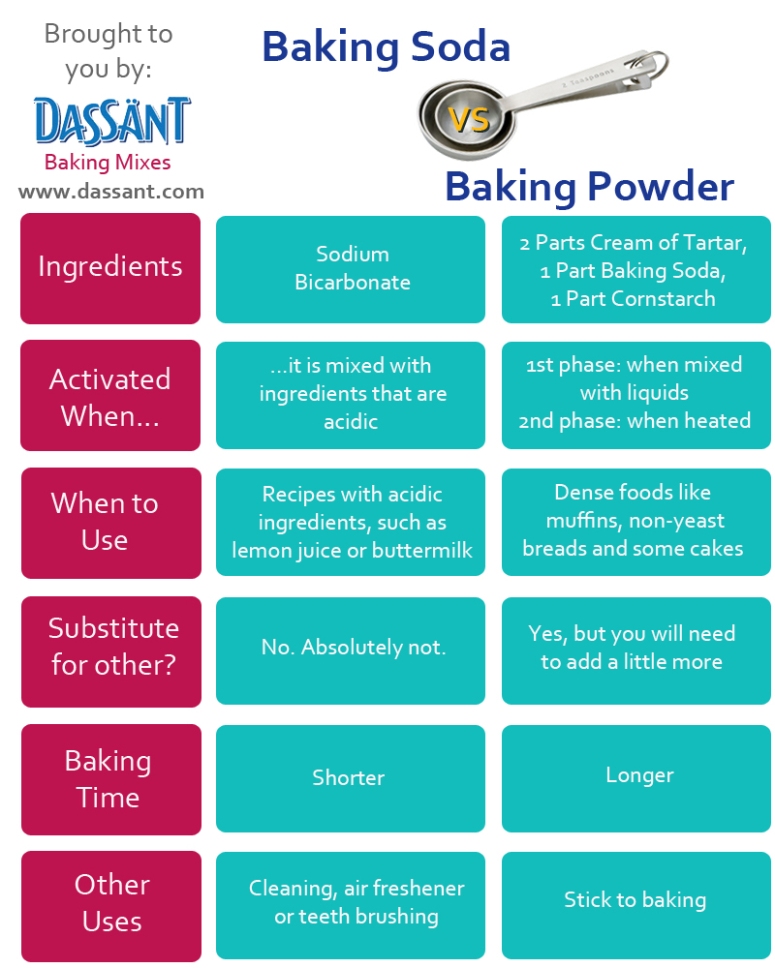
These two have always seemed so similar and it used to confuse me on why every recipe called for specific versions of each, but after doing some research I understand why! Although baking soda is in baking powder, baking powder dilutes it. (This causes more to be necessary when substituting it for baking soda) The properties of baking soda make it good for recipes with acidic ingredients as it prevents things such as heartburn. Baking powder on the other hand adds a fluffiness to food Baking soda’s chemical name is sodium bicarbonate which is also included in Baking powder as said before. If stored in a warm temperature atmosphere, both will eventually lose their potency.
http://culinaryarts.about.com/od/bakingdesserts/a/Baking-Soda-And-Baking-Powder.htm